Designed to Look Viral? Rethinking Endogenous Retroviruses
By Donny Budinsky
Exploring how ERVs shape development, regulate our genes, and challenge the idea they’re just viral leftovers.
It may sound shocking, but it’s true: without endogenous retroviruses (ERVs), you and I wouldn’t be here. These viral-like elements in our DNA aren’t just dead weight — they help build the placenta, regulate our genes, and fine-tune our immune systems. Far from being useless leftovers of infection, ERVs are essential to life itself.
So what exactly are ERVs, and why are some fixed in every person while others are not? Let’s take a closer look.
What Are ERVs?
Endogenous retroviruses are stretches of DNA in our genomes that look like the remains of ancient viral infections.
The question before us is simple but profound: are ERVs accidents of infection written into our genomes, or intentional features crafted to sustain life?
A full ERV typically includes:
- LTRs (long terminal repeats): control switches for gene activity.
- gag: a structural protein.
- pol: an enzyme for copying viral DNA.
- env: a surface protein that allows binding to cells.
At first glance, ERVs look remarkably like retroviruses. That’s one reason many researchers consider them genomic fossils. But this “viral look” is also what makes them functional. For instance, viral envelope-like genes (env) are exactly what allow certain ERVs to fuse cells together in the placenta.
Fixed vs. Unfixed ERVs
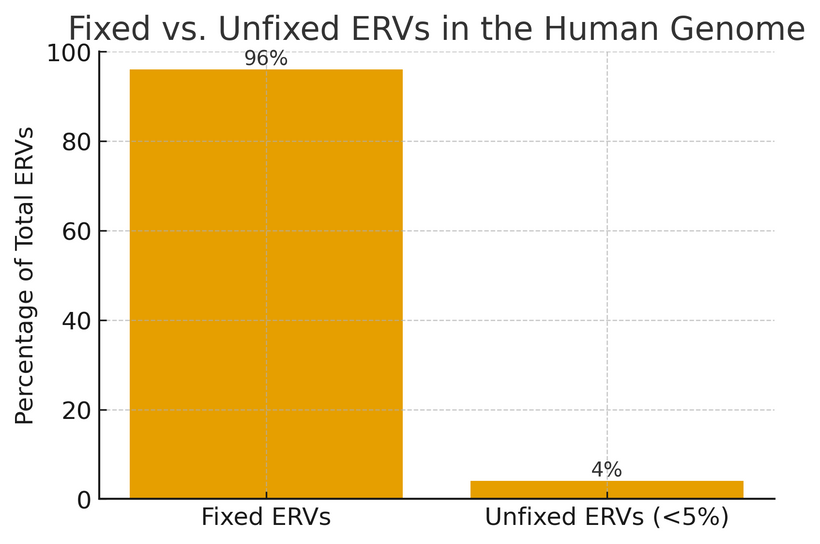
Scientists have found that the vast majority of ERVs in humans are fixed, meaning they are present in every single person worldwide. Only a small fraction are unfixed or polymorphic, showing up in some individuals but not others.
A “polymorphic” ERV is simply one that varies in frequency across the population — in other words, not everyone has it. These unfixed ERVs are rare and usually occur at low frequencies. By contrast, fixed ERVs are universal, stable, and often functional.
Figure 1. Fixed vs. unfixed ERVs in the human population.
This bar graph compares fixed and unfixed ERVs as a percentage of total ERVs. Fixed ERVs dominate the genomic landscape, whereas unfixed ERVs make up only a small fraction. The persistence of fixed ERVs suggests stability and possible function, while unfixed ERVs represent more recent or population-specific insertions.
This difference suggests a possible distinction in origin:
- Fixed ERVs may represent created, functional elements of DNA.
- Unfixed ERVs could be genuine viral insertions that slipped in more recently.
ERVs in Embryological Development
One of the most astonishing examples of ERV function is in placental development. The placenta is a temporary organ that sustains a developing baby, providing nutrients and removing waste. But for it to work, the placenta must fuse tightly to the embryo.
Here’s where ERVs come in: a protein essential for this fusion is encoded by an ERV gene remarkably similar to a viral env gene. In viruses, env allows the virus to bind to host cells. In humans, the same structure is works to bind the placenta to the embryo. Without it, pregnancy as we know it would not be possible.
In other words, without ERVs, we wouldn’t exist. These viral-like elements are essential not only for embryological development but also for shaping the immune system before and after birth. They contribute enzymes, regulatory elements, and mimicry strategies that help the body defend against pathogens. If they did not share sequence similarity with exogenous retroviruses, they would not be able to carry out many of their operational roles.
Not All “ERVs” Are Full ERVs
Here’s something surprising: over 90% of so-called ERVs in our genomes aren’t actually full ERVs. They lack the gag, pol, and env genes. Instead, they exist as solo LTRs — fragments that serve as powerful gene regulators.
These solo LTRs can act as on/off switches, turning nearby genes up or down at the right times. They may not be retroviruses at all, but rather functional stretches of DNA designed to control gene expression.
This complicates the evolutionary argument of “shared ERVs” between humans and chimpanzees. If the majority of these sequences are just solo LTRs, then they may simply represent common design features, not evidence of shared infection history.
Rethinking Origins: The Escape Hypothesis
Conventional evolutionary literature itself has entertained ideas that support this alternative view. Some researchers propose the “escape hypothesis” — that viruses may have originated from host genomes. In this model, genetic elements inside early cells “escaped” and later evolved into infectious retroviruses.
- “Viruses might have come from broken pieces of genetic material inside early cells. These pieces were able to escape their original organism and infect another cell. In this way, they evolved into viruses.” (Let’s Talk Science, n.d.)
- “The ‘escape hypothesis’ suggests that viruses were once part of the genetic material of host cells but escaped cell control and later evolved by pickpocketing genes via horizontal gene transfer.” (Nasir et al., 2012)
This raises a provocative question: if retroviruses require a host to replicate, then hosts must have existed before retroviruses. Could ERVs have been designed within genomes first, with harmful retroviruses later emerging from these created elements?
A Testable Hypothesis
This line of thought leads to a testable hypothesis:
- Fixed ERVs may be designed units of DNA function, crafted to regulate development, immunity, and cellular identity.
- Unfixed ERVs may represent true endogenization events — accidental insertions from infectious retroviruses.
If this hypothesis holds, it would mean ERVs are not ancient viral fossils but engineered elements that only look viral because they must mimic viruses to carry out their roles.
Looking Ahead: What Future Articles Will Explore
This article has laid the foundation for a testable hypothesis: fixed ERVs may be created features, while unfixed ERVs could be true viral insertions. But this is just the beginning.
In future articles, we’ll explore how unfixed ERVs might fit within a historical framework. For example, after the Flood but before the Babel dispersal, true ERV insertions may have occurred. This would make sense, since one of the roles of pre-existing fixed ERVs is to battle exogenous retroviruses and prevent them from becoming endogenized.
Why, then, didn’t these new insertions spread to fixation? The dispersal at Babel may provide the answer. As people scattered across the globe, small, isolated groups carried different ERV insertions with them. This would have limited fixation and slowed its progress. Some ERVs may have reached relatively high frequencies in certain groups before dispersal, but never became fixed worldwide.
This possibility opens the door to future research — and future discussion. Could population history, combined with the functional roles of ERVs, explain the distribution of unfixed elements we see today? Stay tuned as we continue to test and refine this hypothesis.
Key Takeaways
🧬 ERVs are essential — They’re not junk DNA; they play vital roles in placental development, gene regulation, and immune defense.
📉 Most “ERVs” aren’t full retroviruses — Over 90% are just solo LTRs, functioning as regulatory DNA rather than true viral fossils.
👥 Fixed vs. unfixed matters — Fixed ERVs are universal and functional, while unfixed ERVs are rare insertions that look more like genuine infections.
🎯 Design hypothesis — Fixed ERVs may have been created as purposeful features, while unfixed ERVs could represent real viral accidents.
❓ The bigger question — Are ERVs accidents of infection, or intentional features crafted into our genomes to sustain life?
Recommended Reading & References
- Badarinarayan, S., & Sauter, D. (2021). Switching Sides: How Endogenous Retroviruses Protect Us from Viral Infections. Journal of Virology, 95(12), e02299-20. https://doi.org/10.1128/JVI.02299-20
- Nasir, A., Kim, K. M., & Caetano-Anollés, G. (2012). Viral evolution: Primordial cellular origins and late adaptation to parasitism. Mobile Genetic Elements, 2(5), 247–252. https://doi.org/10.4161/mge.22797
- Let’s Talk Science. (n.d.). Where Did Viruses Come From? Retrieved from: https://letstalkscience.ca/educational-resources/stem-in-context/where-did-viruses-come
- Budinsky, D. (2022). The Endogenous Retrovirus Handbook: Dismantling the Best Evidence for Common Descent.